CLL Advocates NZ Newsletter Issue 1
CLL Advocates NZ Newsletter Issue 1
Welcome to our first CLL Advocates newsletter. Like many organisations, we weren’t able to accomplish very much over the lockdown period. We did, however, manage a Trustees’ teleconference to shape up our strategy for the next 12 months. One of the proposals made at that meeting was a monthly newsletter, so here is the first edition.
A huge number of lay and medical articles have been written about the COVID pandemic, and, as you may have seen on our website, we’ve published a number relevant to people living with CLL. See them here. One of particular interest was a consensus statement by Australasian haematologists on management of blood cancers in the COVID pandemic (published in May’s Internal Medicine Journal). It noted that, with a mean age of diagnosis of about 70 years, CLL patients are already likely to be in a high-risk group simply because of age. Advice specifically directed to CLL patients was:
- to delay planned therapy where possible
- to consider using oral agents over IV medications so as to avoid exposure to higher risk environments such as hospital chemotherapy units, for treatment of both initial therapy, and for relapsed/refractory disease (see our recent letter to Pharmac on this matter).
New Zealand has done extraordinarily well at this stage to contain the virus, though it should be noted that vaccination, if successfully developed, may present further challenges for CLL patients.
The CLLANZ Trustees’ meeting agreed that information/education initiatives on CLL should be high on our priority list. In this regard, a review of the current management of CLL by Dr Gillian Corbett, haematologist and trustee, has been published on our website. A detailed CLL patient information booklet is also now close to sign-off and will be published shortly.
In October this year we will be staging a half day or evening combined meeting/seminar on CLL in NZ with the Leukaemia & Blood Cancer (LBC) group, with leading NZ CLL specialists. This will be ‘in person’ in Auckland and also online on Zoom so that people from around the country can join in the discussion and if desired put questions to the speakers. Details will follow soon.
To ensure we reach as many people as possible who have an interest in CLL, we encourage you to become a ‘friend’ of CLL Advocates NZ – by signing up to our newsletter here and/or joining our private Facebook group. You can apply to join the group here.
More details of anticipated activities will follow in subsequent newsletters.
Meanwhile, now that we have passed the winter solstice, and are becoming adjusted to the new ‘normal’, stay well, physically, mentally and spiritually. And please remember that as well as our advocacy role, we want to be a source of knowledge and support for New Zealanders living with CLL, and their families and supporters. To help us achieve this we would welcome and appreciate your feedback, and your thoughts on how we can best achieve our mission.
With best wishes
Neil Graham FRACP, FRCP
Executive Director
CLL Advocates NZ
Three-Drug Combo Promising Against High-Risk CLL
Three-Drug Combo Promising Against High-Risk CLL
For patients with high-risk chronic lymphocytic leukemia (CLL), first-line therapy with a triple combination of targeted agents showed encouraging response rates in the phase 2 CLL2-GIVe trial.
Among 41 patients with untreated CLL bearing deleterious TP53 mutations and/or the 17p chromosomal deletion who received the GIVe regimen consisting of obinutuzumab (Gazyva), ibrutinib (Imbruvica), and venetoclax (Venclexta), the complete response rate at final restaging was 58. 5%, and 33 patients with a confirmed response were negative for minimal residual disease after a median follow-up of 18
Chemotherapy-Free Regimen Consisting of 3 Novel Agents for Previously Untreated, Poor Prognosis CLL
Chemotherapy-Free Regimen Consisting of 3 Novel Agents for Previously Untreated, Poor Prognosis CLL
Nearly 60% of evaluable patients with previously untreated, chronic lymphocytic leukemia (CLL) characterized by chromosomal deletion of 17p (del[17p]) and/or a deleterious TP53 mutation treated with obinutuzumab, ibrutinib, and venetoclax triplet therapy achieved either complete remission (CR) or a CR with incomplete recovery of the bone marrow, according to results of a single-arm, phase 2 study presented during the Virtual Edition of the 25th European Hematology Association (EHA) Annual Congress.
While less than 10% of patients diagnosed with CLL have disease characterized by del(17p), this biomarker is present in up to half of patients with refractory disease.2 Hence, there is an unmet need for efficacious and safe treatment regimens for this subgroup of patients. Furthermore, compounding this need is the finding that TP53 mutations, another biomarker of poor prognosis, have been reported to be present in approximately 90% of patients with disease characterized by del(17p) although only about 40% of those with TP53-mutant CLL also have del(17p).An unmutated IGHV gene is another risk factor for poor prognosis in the setting of CLL.2
Dr. Flinn on the Role of BTK Inhibitors in CLL
Dr. Flinn on the Role of BTK Inhibitors in CLL
This story was originally published on OneLive
Ian W. Flinn, MD, PhD, discusses the role of BTK inhibitors in chronic lymphocytic leukemia.
Ian W. Flinn, MD, PhD, director of the Blood Cancer Research Program, Sarah Cannon Research Institute, discusses the role of BTK inhibitors in chronic lymphocytic leukemia (CLL).
BTK inhibitors such as ibrutinib (Imbruvica), acalabrutinib (Calquence), and zanubrutinib (Brukinsa) have been transformative in the treatment of patients with B-cell malignancies such as CLL, mantle cell lymphoma, marginal zone lymphoma, and Waldenström’s macroglobulinemia, says Flinn.
In CLL specifically, BTK inhibitors have become a prominent frontline standard of care, adds Flinn.
Though, it is important to acknowledge that the toxicities that are associated with ibrutinib, including arthralgias, atrial fibrillation, and, though rare, significant bleeding, often lead to treatment discontinuation in patients with CLL.
'CLL flares’ reported after stopping ibrutinib
'CLL flares’ reported after stopping ibrutinib
Patients with ibrutinib-resistant CLL should remain on the BTK inhibitor until immediately before starting their next therapy, Australian haematologists have advised. In a Commentary article in Leukemia & Lymphoma, Dr Chloe Tang and Associate Professor Constantine Tam wrote that ‘CLL flares’ – accelerated CLL progression after ceasing ibrutinib – can be difficult to distinguish from …
Durable disease control achieved with BTKi salvage therapy in patients with CLL
Durable disease control achieved with BTKi salvage therapy in patients with CLL
Patients with chronic lymphocytic leukaemia (CLL) who progress on venetoclax can achieve durable disease control with Bruton tyrosine kinase inhibitor (BTKi) salvage therapy, new Australian research reveals. The study included 23 patients with refractory/relapsed CLL who received a BTKi (ibrutinib [n=21], zanubrutinib [n=2]) after ceasing the BCL2 inhibitor venetoclax due to progressive disease. After initiating …
To continue reading click here.
A current overview of medications in the treatment of CLL in New Zealand
A current overview of medications in the treatment of CLL in New Zealand
A talk presented to the CLL Advocates NZ Board of Trustees on 7 May 2020 by Dr Gillian Corbett MBChB, FRACPath, MRCP, FRACP, Trustee, CLL Advocates NZ https://clladvocates.nz/about-us/trustees/
Not all CLL patients need treatment. Treatment is offered for progressive disease, increasing lymphadenopathy, anaemia, and/or low platelets, as well as rapidly increasing lymphocyte count.
Patients with mutated heavy genes (about 55%) generally carry a better prognosis than those with unmutated heavy genes. This testing is unfortunately unavailable in NZ.
First line treatment
In New Zealand, FCR (fludarabine, cyclophosphamide, rituximab) is standard treatment for younger patients with CLL, up to around 70-75 years. Fludarabine and cyclophosphamide are generally given orally, once per month for up to 6 courses, and rituximab is given as an intravenous infusion once a month. This treatment is very successful for patients with mutated heavy chain genes, and some may potentially be cured. However those with unmutated heavy chain genes do not respond so well and frequently relapse early. A study has shown that such patients respond well to ibrutinib with a better outcome than FCR. However ibrutinib is not funded to treat CLL in New Zealand.
For older patients (70-85 years) the funded first line treatment is bendamustine and rituximab. Each is given by infusion once per month for up to 6 months. This treatment has been shown to be not so effective for younger patients, for whom FCR is preferred, but is better tolerated by older patients
For older patients or those unfit for bendamustine and rituximab, chlorambucil and obinutuzumab is the funded treatment. Obinutuzumab is a more potent antibody than rituximab and has been shown to be more effective in combination with chlorambucil in these patients.
Venetoclax is funded as first line treatment for patients with del17p chromosomal change. These patients do not generally respond well to the other funded treatments. It targets BCL2 which results in an increased rate of CLL cell death. It is given orally and is generally well tolerated but in some patients with bulky disease it may cause tumour cell lysis which can result in kidney damage. Such patients may require hospitalisation for intravenous fluid and supportive care.
Second line treatment
Patients who relapse within 3 years of previous treatment are eligible for venetoclax and rituximab. The venetoclax is to be used for up to 24 months. It is given orally.
Research
There are studies overseas of combining ibrutinib and venetoclax treatment with a view to achieving negative minimal residual disease and possibly cure of CLL. The results of these studies are awaited.
Ibrutinib is a Bruton’s tyrosine kinase (BTK) inhibitor which interferes with CLL cell growth. It is effective in the majority of de novo and relapsed patients with CLL. It is not known to cure CLL. It is generally well tolerated. Other BTK inhibitors that are more targeted are being trialled eg acalabrutinib and zanubrutinib. Tauranga and Waikato Hospitals are part of an international clinical trial comparing ibrutinib to zanubrutinib in relapsed CLL.
Other areas of research are in the development of immune therapies eg CAR-T cells to target CLL cells.
Study Findings Support Long-Term Efficacy and Safety of Acalabrutinib in R/R CLL
Study Findings Support Long-Term Efficacy and Safety of Acalabrutinib in R/R CLL
Long-term follow-up of a phase 1/2 study demonstrated durable efficacy and safety of acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic leukemia (CLL/SLL), according to results published in Blood.1
Acalabrutinib, an oral selective Bruton tyrosine kinase (BTK) inhibitor, was approved in November 2019 by the US Food and Drug Administration (FDA) at a dose of 100 mg twice daily for the treatment of adult patients with CLL/SLL.2
This analysis included updated results from the initial phase 1/2 study of acalabrutinib in an expanded cohort of 134 adult patients with relapsed/refractory CLL/SLL with a median age of 66 years (ClinicalTrial.gov Identifier: NCT02029443) followed for a median of 41 months. While patients initially received acalabrutinib at a dose of either 200 mg once daily or 100 mg twice daily, only the latter dose of acalabrutinib was administered following a study amendment in May 2015 based on results of pharmacodynamics studies.
At data cutoff, more than half of study patients were still receiving acalabrutinib, with 21% of patients discontinuing acalabrutinib therapy due to progressive disease.
Overall response rate (ORR), including patients achieving a complete response, a partial response. or a partial response with lymphocytosis (PRL) was 94%, with PRL as best response in 6% of patients. Of note, ORR was 90% or higher in the subgroups of patients with disease characterized by del(17)(p13.1), del(11)(q22.3), unmutated immunoglobulin heavy variable (IGHV) genes, and complex karyotype characterized by 3 or more abnormalities. In addition, median duration of response (DOR) was not reached, with 45-month DOR estimated at 63% for those with PRL or better.
Median progression-free survival (PFS) for the overall study population was not reached at the time of the analysis, with 45-month PFS estimated at 62%. In the subgroups of patients with disease characterized by del(17)(p13.1), unmutated/mutated IGHV, complex karyotype, and del(11)(q22.3), median PFS was 36 months, not reached, 33 months, and not reached, respectively.
Regarding safety, grade 3 or higher adverse events occurred in 66% of patients, including neutropenia, pneumonia, hypertension, anemia, and diarrhea in 14%, 11%, 7%, 7%, and 5% of patients, respectively.
Eleven percent of patients discontinued acalabrutinib as a result of one or more adverse events. Adverse event-related death was reported in 7.5% of patients, with half of these attributed to pneumonia.
Atrial fibrillation of any grade occurred in 7% patients, with approximately 3% experiencing atrial fibrillation of at least grade 3. Major bleeding classified as grade 3 or higher, serious, or involving the CNS, was reported in 5% percent of patients.
“Notably, acalabrutinib with follow-up beyond 4 years has shown a decreased frequency of [adverse events] over time and no long-term safety issues to date,” the study authors noted.
In their concluding remarks, they further commented that “this updated and expanded study confirms the efficacy, durability of response, and long-term safety of acalabrutinib, justifying its further investigation in previously untreated and treated patients with CLL/SLL.”
References
- Byrd JC, Wierda WG, Schuh A, et al. Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: updated phase 2 results. Blood. 2020;135:1204-1213.
- Acalabrutinib (Calquence®) [package insert]. 2019. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2019.
Originally published on Cancer Therapy Advisor
Coronavirus: Separating Fact From Fiction for CLL Patients
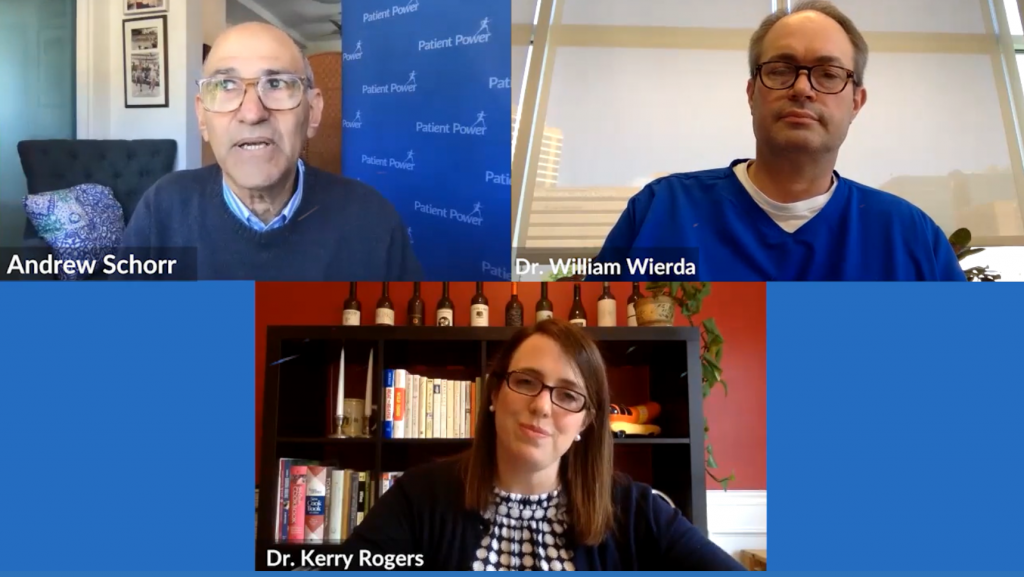
Coronavirus: Separating Fact From Fiction for CLL Patients
“We’re learning every day, and we’re modifying and adjusting based on what we learn,” says Dr. William Wierda, from The University of Houston MD Anderson Cancer Center, as new information continues to come to light on the novel coronavirus.
Can cancer medicines be used to treat COVID-19? Does the effect of the virus vary by CLL subtype? Is ibrutinib (Imbruvica) being mixed with Coca-Cola? Watch to hear expert perspectives on questions like these and more.
With much misinformation circulating online, Dr. Wierda and Dr. Kerry Rogers, from The James Comprehensive Cancer Center at OSU, joined Patient Power to help chronic lymphocytic leukemia patients separate fact from fiction and explain how care is being modified during the pandemic.
This program is sponsored by Pharmacyclics and Janssen. These organizations have no editorial control, and Patient Power is solely responsible for program content.
Click the image to view this video.
Originally published on Patient Power
COVID-19's Impact on How Patients with CLL, Myeloma Have Received Treatment
COVID-19's Impact on How Patients with CLL, Myeloma Have Received Treatment
CURE® recently invited patients, survivors, caregivers, advocates and health care professionals to attend its first-ever live webinar, “Hear from the Experts: COVID-19 & Cancer Care for Patients.”
Sponsored by Janssen and Pharmacyclics, the webinar was designed to provide those affected by chronic lymphocytic leukemia (CLL) and myeloma with information and updates as it pertained to the current landscape of cancer care during the uncertain times of the new coronavirus (COVID-19).
Dr. Saad Usmani, chief of the Plasma Disorders Program and director of clinical research in hematologic malignancies at the Levine Cancer Institute served as the moderator for the webinar. Panelists included:
- Dr. Zainab Shahid, medical director of bone marrow transplant infectious diseases at the Levine Cancer Institute
- Dr. Farukh Awan, director of the Lymphoid Malignancies Program at the Harold C. Simmons Comprehensive Cancer Center at UT-Southwest
- Dr. Ian Flinn, director of the Lymphoma Research Program at Sarah Cannon Research Institute
- Dr. Lee Greenberger, chief scientific officer at the Leukemia and Lymphoma Society
In the first part of this series, Dr. Farukh Awan addresses how the pandemic has affected his practice, as well as how it has impacted how patients with CLL and myeloma have received care.
Awan: We’re all learning. This is something that none of us have experienced before, so we’re learning more and more about the virus with every passing day. There’s more data coming out. There’s a lot of new clinical trials happening, so everything is happening at the same time we are all trying to do the best we can to decrease the spread of this so we can better manage our patients who do get infected and that would not overwhelm the system.
In line with that, every institution has instituted a policy that suits their needs. Fortunately, in Dallas we started with social distancing early and I think we were able to keep the numbers fairly manageable. And yet, we still had to make a lot of changes to the way we practice.
In terms of outpatient management, we have limited the number of visitors that come in with the patient. We try to keep them outside of the clinic area. We also have tried to make the most of our electronic visits so that the least number of patients get exposed to the staff, and vice versa. We have also started utilizing local labs, which are a lot of times not as busy as the oncology clinics and cancer centers. Those labs then give us the results from the blood work that we need. I think we’ve utilized all of these approaches on the outpatient side. We’ve also been fortunate that we have a service for drive-through testing for patients who have active symptoms. We are trying to get them through the drive-through testing and if they are positive, then they’re asked to quarantine at home.
For those who do get really sick and need to go to the hospital, the ER has a system where everybody gets quarantined on arrival. They get tested in a timely manner with a rapid test for their COVID-19 and if they are positive, they are then sent to a specific isolation floor meant for those patients. There are mechanisms in place, both on the outpatient and inpatient side, which have tried to minimize exposure to patients, family members, and staff at the same time.
As far as for patients with chronic lymphocytic leukemia (CLL) who are concerned, a lot of our patients can afford to wait a little bit to start their new treatment. For patients who are not actively getting treated, we do plan on delaying their treatment until at least we have some idea of better treatment modalities and if their symptoms can be managed by other approaches. For those who are on active treatment, we are trying to continue those treatments while trying to minimize their return visits and we’re trying to do that electronically as much as possible, either via telephone calls or through a video visit. Either option is being utilized so that patients can limit their back and forth to the clinic.
We know that our patients with CLL are especially at a high risk of getting infections. Infection-related complications are one of the leading problems in our patients. We expect that our patients would be at an extremely higher risk and that’s why we are also advising that they socially distance themselves from any known patient or persons who might have the virus. We are now also learning that there is a fairly large group of patients, or people in the community, who would have asymptomatic disease and they can potentially spread the disease to a lot of other people without even knowing that they have the virus, and I think that’s very scary for our patients since they are immunocompromised at baseline, even without treatment.
It’s a unique challenge. We are recommending that all our patients with CLL practice social distancing, minimize exposure, and even if they don’t have to go out for groceries they shouldn’t. I think if we can do that over the next few months, hopefully, we can right this and hopefully we will get our patients through it without having any serious complications.
On the other hand, some patients with lymphoma can wait to start treatment, some of them cannot. In some aggressive lymphomas, the treatments need to be started right away. For those patients who need to start treatment right away we are following them very closely for any symptoms. If they exhibit any symptoms, like fevers or a cough or any upper or lower respiratory tract symptoms, we are testing them before we give them any chemotherapy. If somebody is positive for the virus, we are not using chemotherapy for those patients and we are trying to delay chemotherapy as much as possible.
For patients already on treatment and need to be admitted to the hospital we are screening them with a test 48 to 72 hours prior to their admission to the hospital, and once they’re negative, they can then start their treatments. We’re trying to use all these resources that are available to us to try to minimize the risk, again, to the staff and to patients and to prevent any complications that might happen in the future.
Finally, I’d like to make a point regarding stem cell transplants and CAR T. These can be potentially curative options for our patients. We also realize that a lot of our patients will get extremely sick during that process. So, we have multiple challenges with regards specifically to stem cell transplants and CAR T. We’ve also learned that the Red Cross and blood banks are being stretched and that their resources are limited. The number of donors is limited. Since there aren’t a lot of donors like we normally would have, getting access to blood products can be a challenge for our transplant patients, especially if they need blood transfusions and platelets.
We’re trying to very specifically look at all the factors for every patient who is undergoing an autologous transplant, or a CAR T cell treatment and we are trying to select the patient who absolutely needs to proceed with it. We are taking that patient to transplant and are employing a multitude of tests at various time points and making sure there are no visitors during their hospital stay, and as much as possible we are ideally using electronic visits for follow-up to minimize exposure.
Have we done transplants in the last few months? Absolutely. We’ve also done CAR Ts and we have successfully and safely gotten patients through it, but it’s challenging. It can be done but we have to be extremely careful that we minimize exposure to our patients and hopefully we can all get through this.
Originally published on Cure