Uncommon Mutation in CLL Associated With More Favorable Prognostic Factors
Uncommon Mutation in CLL Associated With More Favorable Prognostic Factors
This article was originally published by Cancer Therapy Advisor
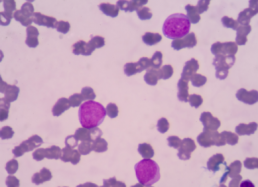
Patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) who had an MYD88 L265P mutation were uncommon and had distinct features, reported a cohort study in Blood Cancer Journal.
Study researchers identified 56 (3.1%) patients with CLL/SLL who had MYD88 mutations among 1779 who underwent mutational analysis. The study also included 100 patients who did not have MYD88 mutations to serve as a control group.
Among patients with MYD88 mutations, 38 (68%) had the L265P mutation and 18 (32%) had non-L265P mutations, which included 10 with V217F and 4 with M232T. Among the remaining patients with non-L265P mutations, 2 had a single-nucleotide mutation, 1 with S219C and the other with A6fs, and 2 had concurrent mutations, 1 with N291S/T294P and the other with F252I/M232T.
No morphological differences were seen, as all patients had a typical CLL morphology. In general, patients with non-L265P mutations had similar features to patients with wild-type MYD88.
Targeted Molecular Therapies for the Treatment of Chronic Lymphocytic Leukemia
Targeted Molecular Therapies for the Treatment of Chronic Lymphocytic Leukemia
This article was originally published by Hematology Advisor
Chronic lymphocytic leukemia (CLL) presents as an accumulation of monoclonal, mature, CD5+ B-cells in secondary lymphatic organs where cancer cells engage in molecular and cellular interactions disrupting the adaptive immune response. Promising advances in therapy options for patients with CLL, targeting these molecular interactions, were discussed in a review published in The New England Journal of Medicine.1
Since the introduction of these novel therapeutics, the use of chemoimmunotherapy has drastically declined. “We have moved away from using chemotherapy for most patients, just over the last 5 years”, Jan Burger, MD, PhD, of the department of leukemia at the University of Texas MD Anderson Cancer Center in Houston, told Hematology Advisor.
“We now have good alternatives to chemotherapy that, in the end, have better survival rates for our patients” Dr Burger, who is the author of the review paper, continued. “The reason why we were able to make this big step forward in treatment was to some extent [serendipitous]. These new kinase inhibitors became available at a time when there was more basic research which emphasized how important these molecules are in leukemia cells.”
Which Drug Therapy for CLL, Time-Limited or Continuous?
Which Drug Therapy for CLL, Time-Limited or Continuous?
This article was originally published by Medscape
About two thirds of patients who are diagnosed with chronic lymphocytic leukemia (CLL) are initially managed with a ‘watch-and-wait’ approach, but other one third of patients will need to be started on drug therapy straight away. But with which regimen?
The standard therapy to date has been time-limited chemoimmunotherapy, and this still has a key role to play in certain cases. But evolving continuous treatments and evidence on the array of factors that can influence response are making decision-making more individualized than ever, said Matthew S. Davids, MD, MMSc, director of clinical research in the Division of Lymphoma at Dana-Farber Cancer Institute and associate professor of medicine at Harvard Medical School, Boston, Massachusetts.
“Continuous versus time-limited treatment discussions are long discussions now and should be individualized to particular patients and their comorbidities,” said Davids.
Davids was speaking at a “How I Treat” session at the recent American Society of Hematology’s (ASH) Meeting on Hematological Malignancies, which was held virtually because of the COVID-19 pandemic.
Key Factors to Consider
Key factors in determining the most effective approach are the patient’s TP53 and immunoglobulin heavy chain (IGHV) gene mutation status, Davids said.
For instance, the traditional choice for frontline care for CLL has been standard chemoimmunotherapy (eg, fludarabine, cyclophosphamide and rituximab [FCR]), with a typical treatment duration of 6 months. However, the response effects appear most durable in younger patients with mutated IGHV and intact TP53.
As evidence, Davids cited data from a long-term follow-up of one of the original FCR studies in 300 patients, which found that about 60% of patients had long-term disease-free survival.
EU approves Imbruvica plus rituximab in first-line CLL
EU approves Imbruvica plus rituximab in first-line CLL
This article was originally published by The Pharma Letter
Johnson & Johnson (NYSE: JNJ) and AbbVie (NYSE: ABBV) have secured approval for a broader label for the BTK blocker Imbruvica (ibrutinib) in Europe.
The decision extends the approved indication in chronic lymphocytic leukemia (CLL) to include first-line treatment in combination with the targeted biologic therapy rituximab, and comes months after the US regulator provided approval in the same indication.
The decision is based on data from the Phase III E1912 study, which provides a favorable comparison with the established chemo-immunotherapy regimen fludarabine, cyclophosphamide and rituximab (FCR).
Profitable partnership
First approved in 2013, Chicago-based AbbVie (NYSE: ABBV) is jointly developing and commercializing Imbruvica with J&J’s pharma arm, Janssen.
The firms are in an equal partnership in developing the drug, after AbbVie acquired the original development partner Pharmacyclics for $21 billion in 2015.
The new approval in CLL represents the 11th approval overall for the product, and the sixth approval for Imbruvica in this cancer type.
4 October - CLL Treatments Today: What Should I Know?
Online webinar: CLL Treatments Today: What Should I Know?
Virtual town meeting - 4 October 5am (NZST)
Overview
Join this virtual town meeting for chronic lymphocytic leukemia (CLL) patients and family members on Saturday, October 3, 2020 starting at 9 am PT/12 pm ET. This 3-hour program will take you through a CLL 101, 201 and 301 class hosted by CLL patient advocates and experts from Cleveland Clinic and University Hospitals Cleveland Medical Center. All attendees will receive a special Powerful Patient face mask.
We want you to be part of this event! Send us your best selfie, or a picture of you with your family, or a pet –anything that tells us who you are! You might just see it on the screen! Please send it to cll@patientpower.info. By sending it to us, you consent to its use in the CLL town hall recorded content and live stream.
Agenda
12:00-12:30 pm ET- CLL 101 – Information for the newly diagnosed. Learn about the stages of CLL, how to talk to your friends and family, and what to ask your doctor.
12:30-12:45 pm ET- CLL and COVID-19 – Is it safe to start or resume treatment right now? Find out how the coronavirus pandemic is impacting CLL patients.
12:45- 1:30 pm ET- CLL 201 – Learn about the current CLL landscape, including treatment options, side effects and the significance of minimal residual disease (MRD) testing.
1:30-1:45 pm ET – Break
1:45 -2:30 pm ET- CLL 301 – Experts discuss the future of CLL treatment, including FDA approvals, clinical trials and Richter’s Transformation.
2:30-3 pm ET – Q&A
Clinical Trials Survey 2020
Clinical Trials Survey 2020
Andrew Schorr, Patient Power co-founder, is the virtual keynote speaker to hundreds of people who supervise cancer clinical trials. The event is on September 10th. The event organizers want Andrew to share his perspective – and yours – on participation in cancer clinical trials now, during the time of the pandemic.
Please help Andrew give a powerful speech based on your experiences and thoughts around clinical trials in context of Covid-19.
The survey will just take a couple of minutes of your time. Thanks for helping and feel free to share with other patients!
Acalabrutinib Shows Promising Results for the Treatment of CLL
Acalabrutinib Shows Promising Results for the Treatment of CLL
This article was originally published by Targeted Oncology
Jennifer Woyach, MD, an associate professor in the Department of Internal Medicine at The Ohio State University Comprehensive Cancer Center–James, discusses the rationale and efficacy for the phase 1/2 trial of acalabrutinib (Calquence) in patients with chronic lymphocytic leukemia (CLL).
Woyach explains that acalabrutinib is an inhibitor of Bruton’s kinase (BTK), and it’s a second-generation covalent inhibitor. Similar to ibrutinib (Imbruvica), it binds irreversibly to BTK, but it is more selective than ibrutinib. Ibrutinib inhibits many structurally similar proteins, while acalabrutinib inhibits less, which is important for toxicity. She thinks that some of the toxicity seen with ibrutinib are because of those alternative targets. Some of the toxicities may lessen with acalabrutinib.
In treatment-naïve and relapsed/refractory disease, the data has looked promising with this agent, according to Woyach. In 1 study with 53 months of follow-up for 99 patients with treatment-naïve CLL, about 86% of the patients were still on treatment, which she says is impressive. The event-free survival at 48 months was 90%. This is looking promising with acalabrutinib, which is similar to what has been seen with ibrutinib in this patient population. Although she says it’s difficult to compare trial to trial, it’s good to see that it looks similar to ibrutinib in this setting.
Online webinar: Telling Others About My CLL Diagnosis
Online webinar: Telling Others About My CLL Diagnosis
How do you tell family and friends that you have leukemia? Chronic Lymphocytic Leukemia (CLL) patients often don’t “look sick” so it may be hard for your family, friends and co-workers to know what you are going through. How do you help others understand your condition?
Join us for our next CLL Answers Now program on Wednesday, September 16th at 10:30 am PT/1:30 pm ET as host and CLL patient Michele Nadeem-Baker discusses with social worker Robin Katz from Lurie Cancer Center of Northwestern University communication tips for people living with CLL, and the best way to share details of their condition. They will also discuss how to talk to their loved ones about their diagnosis and give advice to people newly diagnosed.
Send your questions in ahead to cll@patientpower.info.
Wednesday, September 16, 2020 at 10:30 AM Pacific Time (US and Canada)
Can Culturing CAR T Cells With Ibrutinib Improve Their Efficacy in CLL?
Can Culturing CAR T Cells With Ibrutinib Improve Their Efficacy in CLL?
This article was originally published by Cancer Therapy Advisor
Efficacy of chimeric antigen receptor (CAR) T-cell (CAR-T) therapies in chronic lymphocytic leukemia (CLL) have lagged behind the efficacy they appear to have in other hematological malignancies such as acute lymphoblastic leukemia or diffuse large B-cell lymphoma.
There’s a growing recognition that some of the CAR-T shortfalls in CLL are due to an impaired fitness of T cells in CLL patients. Some research has shown, however, that sustained remission of CLL is associated with enrichment of a less-differentiated, early-memory phenotype of T cells in the apheresis product used to make CAR-T therapies.1 Such findings have galvanized interest in approaches to enhance this T-cell population during the immunotherapy manufacturing process as a possible route for overcoming treatment resistance.
Now, a small study in the International Journal of Cancer reported that culturing CLL patients’ T cells with the tyrosine kinase inhibitor ibrutinib — which among other effects, inhibits a pathway involved in T-cell differentiation — boosted the viability, function, and expansion of CLL patient-derived CAR T cells as well as enriching them with a less-differentiated phenotype.2
World Leukemia Day - 4 September 2020
World Leukemia Day - 4 September 2020

World Leukemia Day is on September 4th and we’d love you to join us.
We are looking for people from all around the globe to participate in increasing the awareness of leukemia that is so vital to help improve the lives of patients after diagnosis.
Here are 3 ways to get involved:
1. Share your leukemia story
Encourage others to share their leukemia story on social media and use the hashtags #WLD2020 or #WorldLeukemiaDay. More information is available at www.worldleukemiaday.org
2. Share your ‘spotty selfie’
Dig out your spotty clothes and share your ‘spotty selfie’ on social media with the hashtag #WLD20 on September 4th to show your support.
3. Spread the word
Help raise awareness of the symptoms of leukemia by using our social media graphics and sharing these across your social media platforms along with the hashtags #WLD20 #worldleukemiaday.
You can download all the social media graphics here > https://bit.ly/WorldLeukemiaDay
World Leukemia Day isn’t just about raising awareness, it’s also about education and learning how to spot the signs and symptoms. For some types of leukemia early diagnosis is crucial, if you are experiencing any of the symptoms, speak to a healthcare professional about your concerns.
Find out more about the signs and symptoms here > https://www.worldleukemiaday.org/what-is-leukemia
We look forward to seeing your ‘spotty selfies’ on September 4th.
Your CLL Advocates Network