Help us Help you! Please join one of three CLL focus groups on 3rd or 10th of December on Zoom.
Help us help you!
We’re getting moving on some research to find out what CLL patients would like CLL Advocates NZ to do for them.
Please help us with this by joining one of three focus groups on Zoom to help us understand what issues are of greatest concern and interest to you and how we can help with them.
The sessions will be very friendly, café-style discussions with groups of 6 – 8 people and a professional facilitator, and you’ll be able to join by one simple click on an email we’ll send you. The groups will be held on :
- Saturday 3rd December 10:00am – 11:30am
- Saturday 3rd December 1:30pm – 3:00pm
- Saturday 10th December 10:00am – 11:30am
We have limited resources so we’re very keen to start the year with a fresh agenda focused on what really matters to patients and their whanau.
Please contact Melanie (our admin assistant) on clladvocates@outlook.co.nz with any questions, and to let us know if you can join one of these groups.
The information we gather from these sessions will be strictly confidential to CLL Advocates NZ and you would not need to show your full name online if you prefer.
Please help us to help you!
Dr Gillian Corbett’s submission to Pharmac
On behalf of CLL Advocates Trustees
Dear friends,
Below is Dr Gillian Corbett’s submission to Pharmac on the proposal to fund Ibrutinib, submitted today. We warmly encourage you to make your own submission - send it to consult@pharmac.govt.nz by 4pm on Thursday. It doesn’t need to be long or detailed, but every voice counts!
With thanks
CLL Advocates NZ
27 September 2022
Submission to Pharmac on the proposal to fund ibrutinib for people with Chronic Lymphocytic Leukaemia
Introduction
CLL Advocates NZ (CLLANZ) is a charity set up by my colleague the late Dr Neil Graham to advocate for the optimal treatment for patients with CLL. I am a retired haematologist with a strong interest in the management of CLL, and I am a Trustee of CLLANZ. I write this submission on behalf of the Trustees and friends of our organisation and in the spirit of the objectives Dr Graham set out in establishing it.
A personal note
As some Pharmac personnel may recall, Dr Graham was a passionate advocate for gaining funded access to ibrutinib for CLL patients, and gave a great deal of his time and energy to give voice to the desperate need for this treatment. This included, among many other things, meetings, and correspondence with Pharmac, submissions, a petition, an appearance before the Health Committee, and an appearance before the Independent Pharmac Review Panel, using the ibrutinib saga as an example of many of the things we believed need to be addressed in Pharmac’s funding approval processes.
We are very sorry Dr Graham did not live to hear the news of your proposal to fund ibrutinib, although like us he would have been disappointed with the narrow criteria being proposed.
- For urgent future consideration
I appreciate that your request for feedback concerns the current proposal specifically, and I provide that in II. below. But for the record I ask you to give urgent consideration to widening the availability of ibrutinib to these further categories of patients with CLL as follows:
- a) For the upfront management of older or less fit patients who are ineligible for fludarabine, cyclophosphamide, rituximab (FCR) treatment who do not have del17 or Tp53 mutations.
The RESONATE study as updated recently, Barr et al, Journal of Clinical Oncology, Vol 39, Issue 15 supplement, 2021 compared upfront ibrutinib with chlorambucil in patients with CLL aged >65. With a follow up of 6.5 years the PFS for ibrutinib was 61% compared to 9% for chlorambucil. For many older patients chlorambucil and obinutuzumab is the only option for upfront treatment. Ibrutinib is an oral therapy with generally manageable side effects. More recently in the COVID era it is desirable that patients should be treated as outpatients without the need for intravenous therapy which is required for chlorambucil and obinutuzumab.
- b) The other mode of treatment we support for older or less fit patients is the combination of venetoclax and ibrutinib. We believe that at present this is the optimal approach.
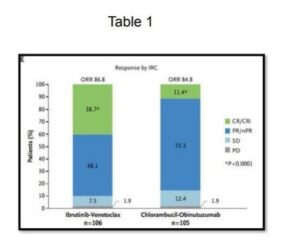
The GLOW Study, Kater et al NEJM Evidence, May 13, 2022, evaluated the efficacy of ibrutinib and venetoclax for older and unfit patients with CLL. The study (N=211, median age 71 years) is randomised evaluating first line fixed duration ibrutinib and venetoclax versus chlorambucil and obinutuzumab in elderly patients, >65 years or younger patients with CIRS >6 or creatinine clearance of <70 ml/min. The patients were randomised to either 3 cycles of ibrutinib followed by 12 cycles of ibrutinib and venetoclax (n=106) or 6 cycles of chlorambucil and obinutuzumab (n=105). This study demonstrated that the combination of ibrutinib and venetoclax demonstrated superior PFS (HR 0.216, CR 0.131 to 0.357 and response, Table 1). This study is for a fixed duration of therapy with, to date, excellent results.
- c) We continue to advocate for ibrutinib for relapsed/refractory patients asan alternativeto venetoclax-containing regimens (as opposed to only after they have had venetoclax); likewise for previously untreated patients with 17p deletion, TP53 mutation, and unmutated IgVH.
- Feedback on Pharmac proposal to fund ibrutinib
As noted above we welcome the recommendation of Pharmac to fund ibrutinib for people with relapsed/refractory CLL following treatment with venetoclax. We see this as a recognition of the value of this drug in the management of patients with CLL.
We submit that:
- Patients who relapse later than 3 years after chemo-immunotherapy or treatment with venetoclax, should be eligible for funded ibrutinib.
We are not aware of any evidence or rationale for denying ibrutinib to this patient group, and believe this inclusion in the Special Authority is an arbitrary restriction that would create inequity among patients in virtually identical situations.
It is possible to repeat FCR in some patients, but this is not always feasible because of age or previous toxicity. Second line options for older patients who relapse after bendamustine and rituximab or chlorambucil and obinutuzumab are very limited.
- Patients who are self-funding ibrutinib on their clinician’s recommendation, or receiving ibrutinib on a compassionate access programme on their clinician’s recommendation, should be eligible to transition to funded treatment.
Specifically excluding these patients in the criteria in the Special Authority is manifestly unfair, affecting as it does the exact patient group for whom the application for ibrutinib was originally intended. The change to today’s CLL treatment landscape could not have been anticipated at the time by clinicians or patients.
We submit that there needs to be a pathway available for these patients to move onto funded ibrutinib at the time the medicine is listed. This could take the form of some type of special request from a clinician or the ability to waive the exclusion in respect of these patients.
We look forward to your response to this submission and are happy to discuss it with you further.
Gillian Corbett MBChB, FRACPath, MRCP, FRACP
Trustee, CLLANZ
Pharmac is proposing to fund Ibrutinib!!!
I’m very pleased to advise that Pharmac has today asked for feedback (Follow this link) on a proposal to fund ibrutinib for New Zealanders with CLL. At last!
It’s seven years since Janssen (the supplier) first applied to Pharmac for funding for ibrutinib for patients with relapsed or refractory CLL.
CLLANZ as a patient advocacy group, and our founder the late Dr Neil Graham in particular, have battled hard over the last few years to highlight the desperate need for this treatment. I’m only sorry that Neil did not live to hear this news.
While it’s great that Pharmac is finally moving on this, it is disappointing that they’ve significantly narrowed the criteria first sought for this treatment. These were to fund it as an alternative to venetoclax-containing regimens, and for previously untreated CLL patients for whom chemoimmunotherapy is inappropriate, and notably those with 17p deletion, and TP53 and unmutated IgVH.
However the Pharmac proposal now up for consultation is to fund ibrutinib for relapsed/refractory CLL patients only after they’ve had venetoclax, or for patients where venetoclax is intolerable. So it’s not available as an alternative to venetoclax, an option clinicians have been calling for urgently for some time. Importantly, it also means it won’t be funded for patients who are currently self-funding ibrutinib, or patients receiving it on a compassionate access programme or on a clinical trial. This seems unreasonable and unfair, and we want to seek clarity from Pharmac on what will happen with these patient groups for whom treatment with ibrutinib remains a high priority.
Pharmac is inviting feedback on their proposal until 4pm on Thursday, 29 September. CLLANZ will be providing feedback and we strongly encourage you as patients and whanau to email Pharmac with your own feedback on this to: consult@pharmac.govt.nz. But we feel that it would be more effective to have a coordinated response from CLLANZ friends and supporters on this and propose to come back to you with some guidance, once we have had time to absorb the detail of the proposal and its ramifications.
Let’s not detract from the fact that today’s development is undoubtedly great news, and I’m sure we will all want to support the proposal. But we might also wish to comment on the eligibility restrictions. It may be, with sufficient encouragement and evidence from the CLL community, that Pharmac will consider extending funding of ibrutinib for all relapsed/refractory CLL patients and ideally, as a first line treatment, finally putting us in step with the rest of the world.
As you may be aware, following the highly critical report by the independent Pharmac Review Panel, Pharmac has undertaken to be more open to patient voices, and we want to take this opportunity to be heard!
With best wishes
Dr Gillian Corbett (On behalf of CLLANZ Trustees)
World CLL day
It is World CLL Day today which is held on the first day of Blood Cancer Awareness Month.
On World CLL Day we also wish to send a message of THANKS to those with healthy immunities for considering our community and showing understanding. During the COVID-19 pandemic many people experienced isolation, fear and the difficulty of keeping safe – an insight into the life of a CLL patient.
https://www.wclld.org/about-world-cll-day/
Rachel Smalley: 1,000 kiwis will die while Pharmac takes eight months to make a decision
OPINION: How long does it take you to make a decision about whether or not to buy something?
You’ve got all the information in front of you. You know it’s a good product. It works. You need it. And you’ve got the money to buy it. So how long will it take?
What if I said that every week that passes where you don’t make a decision, 26 New Zealanders will die? What then? Do you think you could focus or add some urgency to that decision?
I’m pretty sure you could.
Why is it, then, that the same level of urgency doesn’t apply to Government – and in particular, to Pharmac, our drug-buying agency?
World_CLL_Day_September_2022
CLL Advocates June Newsletter
24 June 2022


Our Thoughts at Matariki
Mānawatia a Matariki
We feel it’s appropriate on this special day to remember and celebrate the life of the founder of CLLANZ, Dr Neil Graham. Neil was a very generous, energetic, and compassionate human being who gave his time freely to help improve the lives of New Zealanders living with CLL. His work helped create a ‘community’ of CLL patients, through his personal engagement with patients, creation of the ongoing private CLL Advocates Facebook Group, and production of the first dedicated CLL Patient Booklet for New Zealanders. With a relentless focus on advocating for access to CLL treatments, Neil also raised the profile and understanding of CLL among politicians, Pharmac officials and other policy decision makers. He played a vital role in gaining funding for venetoclax.
Looking to the future, as is also appropriate on this day, the fight for better treatments goes on. In particular, after all these years of waiting, we’d like to see funding for ibrutinib for patients in need of it. But at this stage there’s no sign of any progress on that. While we welcome the release (finally!) of the Pharmac Review Panel report, we’re not greatly confident that it will lead to real change in the nature, speed, and transparency of Pharmac’s decision-making processes. After a three-month delay, releasing the report on the day Prime Minister Ardern met President Joe Biden suggests the Government may not have wanted it to get much attention! We have noted a change in Pharmac’s language but are keen to see some meaningful change in their processes.
We’re very pleased to say that CLLANZ Trustee Dr Gillian Corbett (see her bio Dr Gillian Corbett) has agreed to take on the role of Medical Director of CLLANZ.
You can contact her at: trustees@clladvocates.nz
Gillian will be able to lead or advise on our advocacy activities, but we are still looking for help to progress the priorities we outlined in our last newsletter. Please do get in touch with us if you can help in any way.
We hope you’re having a happy Matariki with family and friends, and we send our warmest thoughts and wishes to Neil Graham’s family and our thanks for all he did for New Zealanders living with CLL.
CLLANZ Trustees
Pharmac Report - Final Review
The Pharmac report has been released.
Dr Andrew Little has advised that:
“The panel found Pharmac’s model has delivered significant benefits, but to achieve its purpose these benefits need to be shared more equitably across our communities, especially for Māori and Pacific peoples,” Andrew Little said.
“As a result of this Review, Pharmac will have a much greater focus on improving the health of Māori, Pacific peoples, disabled people and other groups who do not yet share equitably in the benefits Pharmac provides.
“Pharmac has confirmed to me that it accepts the Panel’s findings. Pharmac is committed to making the needed strategic and operational changes, and already has work underway to do this.
“The panel made 33 recommendations and the government agrees in principle with most of them. There is a small number of recommendations where the government takes a different view, for example where the health reforms will address the underlying issues now or in the future,”
What are your thoughts on the review?
Here is the link for the Pharmac Review. Pharmac Review - Final Report
This is the Executive summary: Pharmac-Review-Executive-Summary.pdf
International COVID-19 Blood Cancer Coalition (ICBCC) - Patient Impact Statement and Recommendations
Protecting immunocompromised blood cancer patients during the COVID-19 pandemic
The coalition has prepared a Joint Patient Impact Statement for use in different countries to aid when advocating for the provision of anti-COVID-19 treatment and care for immunocompromised or immunosuppressed (IC/IS) blood cancer patients.
The Statement has to date been endorsed by networks and national organisations of the global patient advocacy community as well as renowned medical societies and representatives from the global clinical community .
There is a total of 71 endorsers to date including CLL Advocates NZ, Dr Gillian Corbett.
CANCER AGENCY REPORT DUPLICATES PHARMAC ROLE
MULTIPLE MYELOMA MEDIA RELEASE
FOR IMMEDIATE RELEASE
28 April 2022
CANCER AGENCY REPORT DUPLICATES PHARMAC ROLE
The Cancer Control Agency’s analysis of the gap between Australia and New Zealand in funding 18 selected medicines with substantial clinical benefit is a welcome way of drawing attention to the plight of NZ cancer patients desperate for treatments that are funded across the Tasman, but not here, says Myeloma NZ Chief Executive Dr Ken Romeril.
“It’s good to see the Agency weighing in on this issue, but their findings are not news to anyone involved in cancer treatment, and this kind of analysis is surely part of Pharmac’s job in evaluating medicines.
Dr Romeril said the release of the report raises a number of important questions:
- Why is it necessary for another government agency within the Ministry of Health to spend what must be a very large amount of money on work that should be done by Pharmac, and might have been better spent on funding medicines?
- How does the release of this report fit with the withholding of the final report of the Independent Pharmac Review Panel?
- What has become of the Review Panel’s report, which must surely examine whether Pharmac’s model and methodology is keeping NZ in step with the rest of the world in terms of access to modern medicines?
- How does this list of 18 ‘gold standard’ medicines line up with Pharmac’s Options for Investment list of 78 medicines?
Dr Romeril said the most important question is what this report might mean for those NZ cancer patients who right now are struggling with desperate and severe unmet need for treatment.
“In that regard a further concern I have about this report is that it reinforces the idea that there are only two types of treatment: curative and life-extending. That is a crude distinction and sows the idea that funding a medicine that extends life by a short time is not worthwhile.
“In fact many cancers have been cured in our lifetime. And for a number of cancers there are cures on the horizon, with life-extending treatments often becoming the bridge that keeps the patient alive and well until the next breakthrough or the cure has arrived.
“Approaches to treating multiple myeloma are a good example of this point. Transformative, life-extending treatments have been available and funded throughout the Western world for several years. And a cure is in fact in sight. But in NZ myeloma is one of the most neglected areas in terms of access to new treatments, with no new treatments having been funded for in NZ for the past 7 years”, said Dr Romeril.
Myeloma patient and young mother Nichola Oakenfull says seeing the Agency’s report just highlights the pain of her predicament.
“It’s pretty demoralising as a patient to read the report talking about curative and non-curative cancers. It feels like we are cast aside because there is no cure for us.
“There’s no cure for diabetes. But we accept the cost of continuing to treat diabetics as the right thing to do. Should we decide not to treat them because they are going to die anyway?
“Overseas, people with my cancer are living long and productive lives because they have access to drugs that manage their cancer for long periods, and when one drug stops working they move to the next option. Here in New Zealand we only have two lines of treatment for transplant eligible patients, and then it’s game over.
“As shown in the report, Australia has carfilzomib, daratumumab and pomalidomide funded for myeloma, while these three are not funded in New Zealand at all. These are not fancy, new, unproven drugs. They are standard of care overseas. People are shocked when I mention in international groups that we don’t have these medicines here in NZ.
“The Malaghan Institute in Wellington is doing amazing work with CAR T-cell trials. I want to be around for when they start using them for myeloma patients.
“I have an eight year old son I need to stay alive for, but I potentially won’t with the currently approved drugs. Knowing that it could all be so different if I lived across the Tasman is honestly heart-breaking, and so unfair”, said Ms Oakenfull.
Dr Romeril said Myeloma NZ was disappointed that blood cancers had not been included in the report, but noted that the Agency intended to produce a separate report on these.
“We will look forward to that with great interest,” said Dr Romeril.
ENDS
For further information:
Dr Ken Romeril
Chief Executive, Myeloma NZ
0274 432 624
Nichola Oakenfull
Patient, and Trustee, Myeloma NZ
027 454 9682
Joy Wilkie
Patient, and Trustee, Myeloma NZ
027 415 5460
Joy.wilkie@icloud.com
contact@multiplemyeloma.org.nz
Myeloma New Zealand
PO Box 25162, Wellington 6011
Registered Charity CC53924
Myeloma New Zealand focuses specifically on multiple myeloma and improving the lives of patients affected by it. Our mission includes campaigning for the best patient care, gaining new, improved treatments, and enabling support for affected families.
Around 360 New Zealanders are diagnosed with this form of blood cancer every year. Myeloma New Zealand wants to provide support and information to help them and their families and loved ones to understand the myeloma ‘journey’ from diagnosis to treatment. We want to help them through the milestones they may encounter along the way and the likely options and choices they may have. But most importantly, we want to help beat this cancer by identifying and supporting strategies and research initiatives that will both improve the quality of life of those living with myeloma and extend their lives.
contact@multiplemyeloma.org.nz
www.multiplemyeloma.org.nz