Dr Gillian Corbett’s submission to Pharmac
On behalf of CLL Advocates Trustees
Dear friends,
Below is Dr Gillian Corbett’s submission to Pharmac on the proposal to fund Ibrutinib, submitted today. We warmly encourage you to make your own submission – send it to consult@pharmac.govt.nz by 4pm on Thursday. It doesn’t need to be long or detailed, but every voice counts!
With thanks
CLL Advocates NZ
27 September 2022
Submission to Pharmac on the proposal to fund ibrutinib for people with Chronic Lymphocytic Leukaemia
Introduction
CLL Advocates NZ (CLLANZ) is a charity set up by my colleague the late Dr Neil Graham to advocate for the optimal treatment for patients with CLL. I am a retired haematologist with a strong interest in the management of CLL, and I am a Trustee of CLLANZ. I write this submission on behalf of the Trustees and friends of our organisation and in the spirit of the objectives Dr Graham set out in establishing it.
A personal note
As some Pharmac personnel may recall, Dr Graham was a passionate advocate for gaining funded access to ibrutinib for CLL patients, and gave a great deal of his time and energy to give voice to the desperate need for this treatment. This included, among many other things, meetings, and correspondence with Pharmac, submissions, a petition, an appearance before the Health Committee, and an appearance before the Independent Pharmac Review Panel, using the ibrutinib saga as an example of many of the things we believed need to be addressed in Pharmac’s funding approval processes.
We are very sorry Dr Graham did not live to hear the news of your proposal to fund ibrutinib, although like us he would have been disappointed with the narrow criteria being proposed.
- For urgent future consideration
I appreciate that your request for feedback concerns the current proposal specifically, and I provide that in II. below. But for the record I ask you to give urgent consideration to widening the availability of ibrutinib to these further categories of patients with CLL as follows:
- a) For the upfront management of older or less fit patients who are ineligible for fludarabine, cyclophosphamide, rituximab (FCR) treatment who do not have del17 or Tp53 mutations.
The RESONATE study as updated recently, Barr et al, Journal of Clinical Oncology, Vol 39, Issue 15 supplement, 2021 compared upfront ibrutinib with chlorambucil in patients with CLL aged >65. With a follow up of 6.5 years the PFS for ibrutinib was 61% compared to 9% for chlorambucil. For many older patients chlorambucil and obinutuzumab is the only option for upfront treatment. Ibrutinib is an oral therapy with generally manageable side effects. More recently in the COVID era it is desirable that patients should be treated as outpatients without the need for intravenous therapy which is required for chlorambucil and obinutuzumab.
- b) The other mode of treatment we support for older or less fit patients is the combination of venetoclax and ibrutinib. We believe that at present this is the optimal approach.
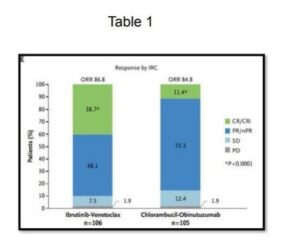
The GLOW Study, Kater et al NEJM Evidence, May 13, 2022, evaluated the efficacy of ibrutinib and venetoclax for older and unfit patients with CLL. The study (N=211, median age 71 years) is randomised evaluating first line fixed duration ibrutinib and venetoclax versus chlorambucil and obinutuzumab in elderly patients, >65 years or younger patients with CIRS >6 or creatinine clearance of <70 ml/min. The patients were randomised to either 3 cycles of ibrutinib followed by 12 cycles of ibrutinib and venetoclax (n=106) or 6 cycles of chlorambucil and obinutuzumab (n=105). This study demonstrated that the combination of ibrutinib and venetoclax demonstrated superior PFS (HR 0.216, CR 0.131 to 0.357 and response, Table 1). This study is for a fixed duration of therapy with, to date, excellent results.
- c) We continue to advocate for ibrutinib for relapsed/refractory patients asan alternativeto venetoclax-containing regimens (as opposed to only after they have had venetoclax); likewise for previously untreated patients with 17p deletion, TP53 mutation, and unmutated IgVH.
- Feedback on Pharmac proposal to fund ibrutinib
As noted above we welcome the recommendation of Pharmac to fund ibrutinib for people with relapsed/refractory CLL following treatment with venetoclax. We see this as a recognition of the value of this drug in the management of patients with CLL.
We submit that:
- Patients who relapse later than 3 years after chemo-immunotherapy or treatment with venetoclax, should be eligible for funded ibrutinib.
We are not aware of any evidence or rationale for denying ibrutinib to this patient group, and believe this inclusion in the Special Authority is an arbitrary restriction that would create inequity among patients in virtually identical situations.
It is possible to repeat FCR in some patients, but this is not always feasible because of age or previous toxicity. Second line options for older patients who relapse after bendamustine and rituximab or chlorambucil and obinutuzumab are very limited.
- Patients who are self-funding ibrutinib on their clinician’s recommendation, or receiving ibrutinib on a compassionate access programme on their clinician’s recommendation, should be eligible to transition to funded treatment.
Specifically excluding these patients in the criteria in the Special Authority is manifestly unfair, affecting as it does the exact patient group for whom the application for ibrutinib was originally intended. The change to today’s CLL treatment landscape could not have been anticipated at the time by clinicians or patients.
We submit that there needs to be a pathway available for these patients to move onto funded ibrutinib at the time the medicine is listed. This could take the form of some type of special request from a clinician or the ability to waive the exclusion in respect of these patients.
We look forward to your response to this submission and are happy to discuss it with you further.
Gillian Corbett MBChB, FRACPath, MRCP, FRACP
Trustee, CLLANZ