CLL forum with Dr Philip Thompson
CLL forum with Dr Philip Thompson
Dr Philip Thompson is a clinician and researcher at the MD Anderson Cancer Centre in Texas. In October 2019, he presented at a Leukaemia Foundation forum on the latest research and directions in CLL management and treatment.
Pharmac funds new treatment option for New Zealanders living with chronic lymphocytic leukaemia
Pharmac funds new treatment option for New Zealanders living with chronic lymphocytic leukaemia
This article was originally published on New Zealand Doctor
- VENCLEXTA® (venetoclax) plus rituximab will be funded from 1 December 2019 for patients with chronic lymphocytic leukaemia who have relapsed within 36 months of previous treatment.
- Every year in New Zealand around 120 people are diagnosed with chronic lymphocytic leukaemia (CLL). It is the most common form of leukaemia in New Zealand.[1]
- VENCLEXTA is based on an Australian research discovery of a protein in the body called BCL-2 that helps CLL cells survive. Blocking this protein helps to kill and reduce the number of these cancer cells.[3]
AbbVie (NYSE: ABBV) New Zealand welcomes PHARMAC’s announcement that patients with chronic lymphocytic leukaemia (CLL) whose cancer has relapsed within 36 months of previous treatment will now have funded access to VENCLEXTA® (venetoclax) in combination with rituximab, from 1 December 2019.[2]
Chronic lymphocytic leukaemia (CLL) is a type of cancer affecting white blood cells called B-cells, and may also involve the lymph nodes. In CLL, the B-cells multiply too quickly and live too long, so that there are too many of them in the blood.[3]
Many people with chronic lymphocytic leukemia have no early symptoms. Those who do develop signs and symptoms may experience enlarged lymph nodes, fatigue, fever, night sweats, weight loss and frequent infections.[4]
Each year in New Zealand around 120 people are diagnosed with CLL, making it the most common type of leukaemia diagnosed in New Zealand.1 Although diagnosed on occasion in adults aged 35-55 years, CLL usually affects people over 60 years of age, and is diagnosed more often in men than women.[5] CLL usually develops and progresses slowly over many months or years.1
VENCLEXTA® (venetoclax) is an oral targeted treatment that works by blocking a protein in the body (“BCL-2”) that helps CLL cancer cells survive. Blocking this protein helps to kill and reduce the number of cancer cells, and may slow the spread of CLL.3
Each year in New Zealand around 120 people are diagnosed with CLL, making it the most common type of leukaemia diagnosed in New Zealand.1
Leading haematologist Dr Robert Weinkove says today’s announcement is a major step forward for New Zealanders who are living with CLL. “Many people with early-stage CLL can be safely monitored by their GP, but most eventually need treatment. It’s vital that we improve access to new targeted cancer medications so we can help New Zealanders with CLL.”
VENCLEXTA was developed as part of a research collaboration between AbbVie, Genentech, a member of the Roche Group of Companies, and the Walter and Eliza Hall Institute in Melbourne, Australia.
AbbVie New Zealand’s General Manager, Andrew Tompkin, commended PHARMAC for recognising the unmet need of CLL patients in our community. “At AbbVie our goal is to provide medicines that make a transformational improvement in cancer treatment and outcomes for patients. Today’s news that the funding of VENCLEXTA means that patients whose CLL has returned have another option where previously their treatment choices were limited.”
[1] https://www.leukaemia.org.nz/information/about-blood-cancers/leukaemia/chronic-lymphocytic-leukaemia/
[2] https://www.pharmac.govt.nz/news/consultation-2019-09-01-venetoclax/
[3] Venclexta Consumer Information (CMI). Version 6. Updated May 2019 https://medsafe.govt.nz/consumers/cmi/v/venclexta.pdf
[4] https://www.mayoclinic.org/diseases-conditions/chronic-lymphocytic-leukemia/symptoms-causes/syc-20352428 Accessed October 2019
[5] MOH Data on File 2019
Ibrutinib May Be More Effective Than Ofatumumab in Chronic Lymphocytic Leukemia
Ibrutinib May Be More Effective Than Ofatumumab in Chronic Lymphocytic Leukemia
This article was originally published on Hematology Advisor
Final results from the phase 3 RESONATE trial (ClinicalTrials.gov Identifier: NCT01578707) suggest that ibrutinib may be more effective than ofatumumab for treating patients with relapsed or refractory chronic lymphocytic leukemia (CLL). The results were published in the American Journal of Hematology.
Earlier results from RESONATE suggested that single-agent ibrutinib, a Bruton’s tyrosine kinase inhibitor administered once daily, has greater efficacy compared with ofatumumab for treating patients with CLL/small lymphocytic lymphoma, including those with high-risk features such as 17p deletion, TP53 mutation, 11q deletion, or unmutated IGHV. For this analysis, the authors reviewed long-term safety and efficacy data.
A total of 195 patients were randomly assigned to receive ibrutinib and 196 received ofatumumab. The median duration of treatment was 41 months (range, 0.2-71.1) with ibrutinib and 5.3 months (range, 0-9.0) with ofatumumab; the planned duration with the latter, however, was 24 weeks. Of the 196 patients receiving ofatumumab, 68% (133) crossed over to the ibrutinib treatment arm.
Median overall follow-up was 74 months. Median progression-free survival (PFS) was 44.1 months with ibrutinib and 8.1 months with ofatumumab (hazard ratio [HR], 0.148; P <.001). PFS was also better in the high-risk patient group that received ibrutinib compared with high-risk patients who received ofatumumab (HR, 0.110).
Overall survival was better in the ibrutinib group (67.7 vs 65.1 months), even after adjusting for crossover (HR, 0.639).
Frequently reported grade 3 or worse adverse events in the ibrutinib group included neutropenia (25%), pneumonia (21%), thrombocytopenia (10%), anemia (9%), hypertension (9%), urinary tract infection (7%), diarrhea (7%), and atrial fibrillation (6%). The prevalence of grade 3 or worse adverse events in patients receiving ibrutinib was 62% in the first year of treatment and decreased to 32% by the sixth year of treatment.
At study termination, 36.9% of patients who received ibrutinib discontinued treatment due to progressive disease, 16.4% due to adverse events, 7.7% due to withdrawal from study, 6.7% due to death, 10.3% due to investigator decision, and 22.1% due to study termination by sponsor. Of the 133 patients who crossed over from the ofatumumab group, 47 were still receiving therapy at study closure.
“These 6-year follow-up results from the RESONATE study confirm the robust and durable efficacy of ibrutinib with extended treatment in patients with relapsed or refractory CLL,” the researchers wrote.
Reference
1. Munir T, Brown JR, O’Brien S, et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma [published online September 11, 2019]. Am J Hematol. doi:10.1002/ajh.25638
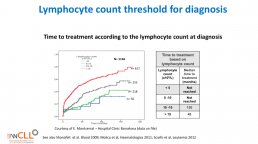
Lymphocyte count threshold for diagnosis
Lymphocyte count threshold for diagnosis
This slide calculates the likely time from being diagnosed with CLL to the commencement of formal treatment for CLL, based on lymphocyte count at diagnosis.
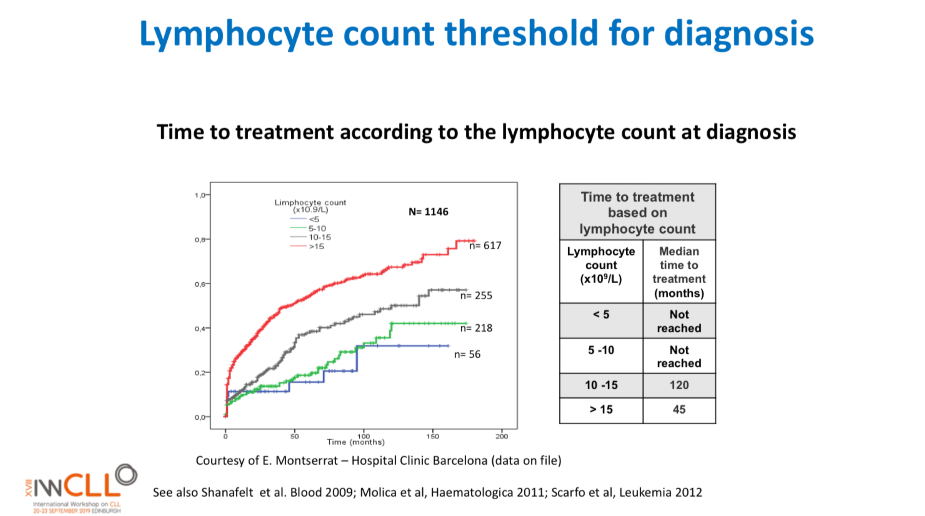
The time from diagnosis to treatment is started is often called the “watch and wait” period. This can be a very stressful time.
This slide uses the patient’s lymphocyte count at diagnosis to predict when treatment with medications will likely start.
The horizontal axis is months from time of diagnosis until treatment with medications is likely to start, and the vertical axis is how likely the patient is at a certain number of months after diagnosis to be on treatment (0.0 = no chance, 1.0 = certain to be having treatment). So if the patient’s lymphocyte count at diagnosis is greater than 15b/L, they have about a 50% chance of being started on treatment 50 months after diagnosis.
Pharmac to respond to CLLANZ petition
Pharmac to respond to our petition at Health Select Committee hearing this week
Go along in person or watch live on Facebook (see details below) as Pharmac presents their oral submission at the Committee’s meeting on Wednesday, 23 October 2019, from approximately 08.30am to 09.50am, at Parliament Buildings in Wellington. They will address all of the following petitions in one submission.
- Petition of Jeffrey Chan: Ask Pharmac to fund Osimertinib
- Petition of Rachel Brown for Ovarian Cancer New Zealand: Fund Lynparza and Avastin for Ovarian Cancer Patients
- Petition of Emma Crowley for Breast Cancer Aotearoa: Breast Cancer Aotearoa Coalition: Fund breast cancer drugs
- Petition of Neil Graham for Chronic Lymphocytic Leukaemia Advocates New Zealand : Fund life-saving treatments for chronic lymphocytic leukaemia
- Petition of Philip Hope for Lung Foundation New Zealand: Ask Pharmac to fund innovative treatments for lung cancer
- Petition of Kenneth Romeril for Myeloma New Zealand: Fund transformative treatments for multiple myeloma patients
- Petition of Janine Yeoman: Lifesaving treatment for people who suffer from Spinal Muscular Atrophy
- Petition of Allyson Lock for the New Zealand Pompe Network: Fund Myozyme for Adults with Pompe Disease
The meeting will be open to the public, and you are welcome to attend in person or watch the live stream of the hearing on the Health Committee Facebook page here.. At this time the exact room the meeting will be held in is not decided, but you will be able to check which meeting room it will be held in closer to the date of the meeting on the Select Committee Schedule webpage here. The meeting room will also be displayed on electronic signboards inside Parliament on the day.
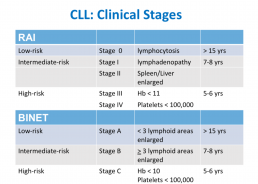
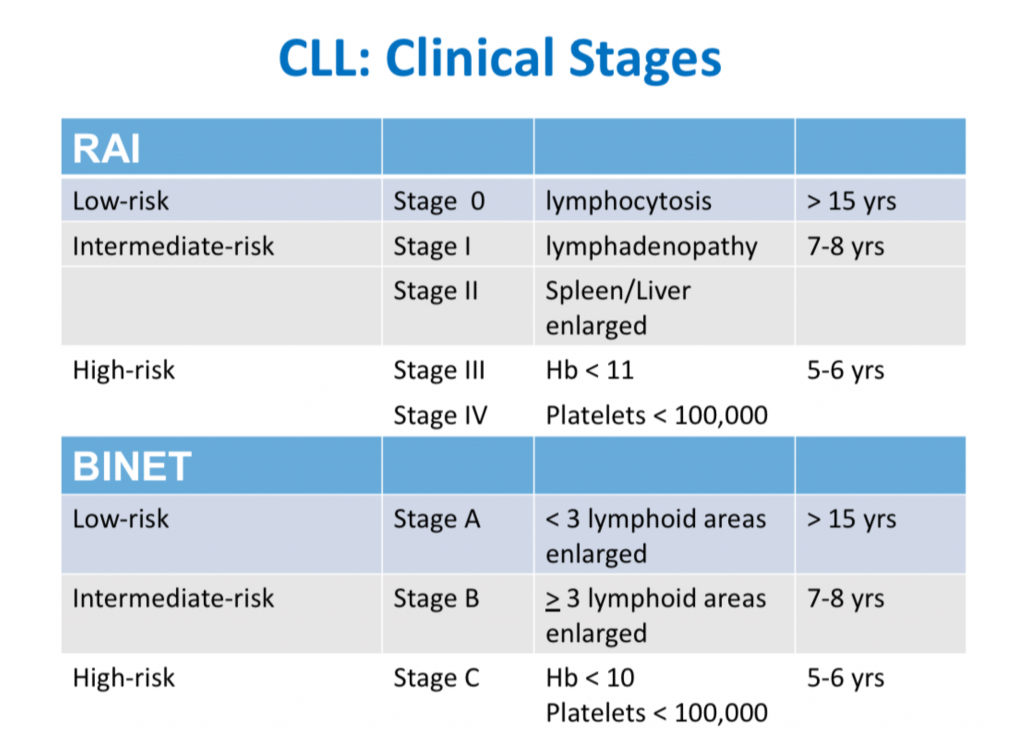
CLL Classification Systems
CLL Classification Systems
This is the third in a series of slides from the recent CLL conference in Edinburgh that may be of interest to CLL patients.
‘Rai’ and ‘Binet’ are two classification systems for CLL disease severity, developed around the same time several decades ago. Rai is a US CLL expert, and Binet is a French expert. Both systems are still used, interchangeably. Some clinicians prefer one over the other. It’s likely that another, more modern classification will be developed in the next few years.
Some explanations:
The right-hand column is known disease duration since diagnosis in the individual patient being staged.
RAI:
Lymphocytosis = increased lymphocyte numbers in the blood.
Lymphadenopathy = increase in lymph node size.
Hb = haemoglobin, a measure of the red blood cells (Hb < 11 is anaemia).
Platelets < 100,000 is a reduced platelet number
BINET:
Lymphoid areas = anatomical areas of lymph node enlargement eg enlargement of nodes under the armpits, in the groin etc.
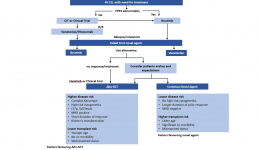
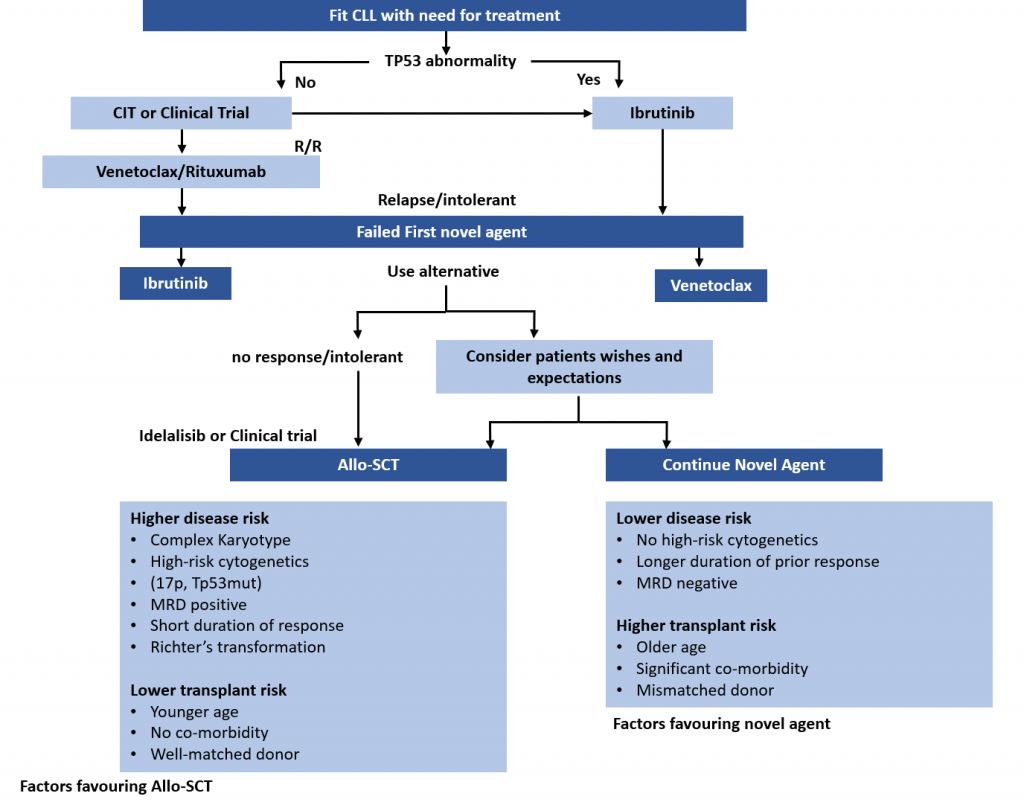
Treatment Pathways for Active CLL
Treatment Pathways for Active CLL
This is the second in a series of slides from the recent CLL conference in Edinburgh that may be of interest to CLL patients.
This summarises current treatment options for active CLL and lists risk factors that would influence treatment decisions.
Abbreviations:
CIT = chemoimmunotherapy, eg FCR (fludarabine, cyclophosphamide, rituximab)
Allo SCT = allogeneic stem cell transplant with stem cells from another matching person – related or unrelated. In practical terms, it is uncommonly done, can cause GVH (graft versus host disease), and is recognised as a potential cure for CLL .
MRD = minimal residual disease, ie remission.
Wake-up call for cancer agency boss Diana Sarfati
Wake-up call for cancer agency boss Diana Sarfati
My colleague haematologist Ken Romeril of Myeloma NZ has an excellent article on stuff today calling out Pharmac and the new cancer agency on their failure to factor blood cancers into their plans.
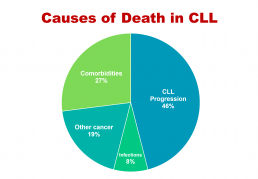
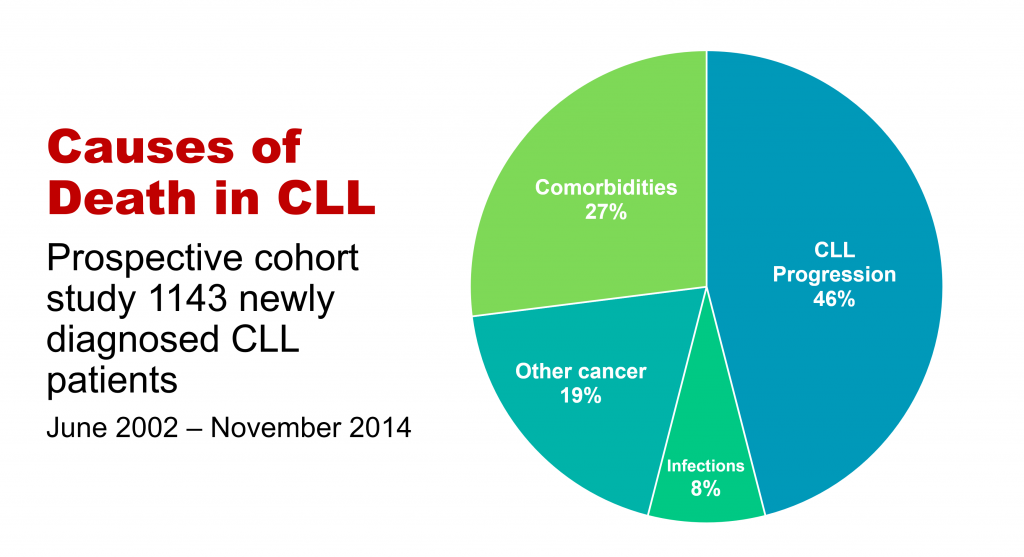
'Take-out messages' from CLL Horizons
'Take-messages' from CLL Horizons
This is the first in a series of slides from the recent CLL conference in Edinburgh that may be of interest to CLL patients.
The important information here is that under half of people with CLL die primarily of that disease, while the rest die of other cancers, infections, or other diseases (comorbidities). So, if you have CLL, it’s important to be checked regularly for other cancers, such as melanoma and other skin cancers, prostate cancer in men, breast cancer in women, and gastrointestinal (oesophageal, stomach, and colon) cancers and lung cancer, in both sexes. This relates to the fact that people with CLL have a reduced ability to control cancer in their bodies because of the impaired immune system that goes with the disease.
If you develop any infection, take it seriously, as if you have CLL you’re at higher risk of a serious episode of infectious disease or a fatal outcome, again because your immune system function is impaired. You may also need more prolonged antibiotic courses, with infective diseases in the context of CLL.
The third group of non-CLL causes of death in CLL, more than a quarter of cases, relates to pre-existing conditions such as heart disease, diabetes, and chronic obstructive pulmonary disease, which either precede or develop during the course of CLL, often independently. This is partly because approximately half of people with CLL are 70+ years old at diagnosis, and partly because a lot of people with CLL, particularly the elderly patients, have a smouldering, relatively stable or only slowly progressive form of the disease. It also makes the point that important comorbidities need to be managed optimally in people with CLL.
Neil Graham
Study of Ibrutinib in CLL Shows a Benefit, but Raises Crucial Questions
Study of Ibrutinib in CLL Shows a Benefit, but Raises Crucial Questions
Efforts to target key mechanisms in the development of chronic lymphocytic leukemia (CLL), one of the most common lymphoid cancers, have led to several new treatments. Among these, ibrutinib emerged as a strong approach for patients who were too frail for aggressive treatment.1 But for healthier patients with CLL, the benefit of this drug remained unknown for a time.
A phase 3 study published in the New England Journal of Medicine showed a benefit for ibrutinib plus rituximab for these more fit patients.2 Although the data help clarify the potential role of ibrutinib in CLL, they also raise difficult questions about the future of this treatment approach.
The standard first-line treatment for otherwise healthy CLL patients 70 years or younger has been a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab (FCR). Patients with immunoglobulin heavy-chain variable region (IGHV)-mutated disease had particularly good results with this treatment: roughly half remained progression-free for up to 8 years after initial treatment. However, substantial toxic effects in the aftermath of treatment, including myelosuppression, risk of myelodysplasia, and infectious complications, have led researchers to explore other avenues of therapy — in particular, ibrutinib.
Ibrutinib inhibits Bruton tyrosine kinase (BTK), a B-cell signaling protein involved in B-cell development, differentiation, proliferation, and survival. After studies showed the drug to be effective for patients with relapsed CLL and then frail patients with untreated CLL, researchers have been eager to investigate its role in first-line therapy for younger patients.
Most studies so far have explored the use of ibrutinib as a monotherapy. This trial, however, led by Tait Shanafelt, MD, of Stanford University School of Medicine, California, combined ibrutinib with rituximab, and compared the regimen with the standard treatment of FCR.
The study began with 529 patients, all with previously untreated CLL. A total of 354 patients, all 70 years or younger, were randomly assigned to receive ibrutinib-rituximab treatment, consisting of 1 cycle of ibrutinib, followed by 6 cycles of ibrutinib-rituximab combined, and then ibrutinib alone, taken indefinitely until disease progression. The 175 patients in the control group received 6 cycles of FCR chemoimmunotherapy. The primary end point was progression-free survival (PFS), with overall survival (OS) as the secondary end point.
Patients in the ibrutinib-rituximab group received 1 dose of 420 mg per day of ibrutinib during day 1 to day 28 of each of the 6 cycles, until either their disease progressed or the side effects became intolerable. Alongside this dosage, they also received rituximab, during cycles 2 through 7. The FCR group received 6 cycles of FCR.
At a median follow-up of 33.6 months, Dr Shanafelt and the team found a benefit for the experimental treatment. At that point, 89.4% of patients in the experimental group had experienced no disease progression, compared with 72.9% in the control group. OS rates echoed the benefit: 98.8% of patients in the experimental group were still alive, compared with 91.5% of patients in the control group.
The researchers wondered if treatment efficacy would be different for patients with the IGHV-mutated CLL. Dividing the existing patients into 2 subgroups, they found that for patients carrying the mutation, the 2 protocols were nearly identical — and in fact, FCR proved 0.3% more effective. For those without the mutation, however, the ibrutinib-rituximab combination was superior to FCR: with 90.7% PFS at 3 years, compared with 62.5%, respectively. Patients in the experimental group without IGHV-mutated disease also experienced fewer serious infections.
Researchers concluded that treatment with ibrutinib-rituximab was superior to FCR with regard to both PFS and OS. They reported a 65% lower risk of progression and an 83% lower risk of death. This superior PFS was also present in high-risk subgroups, including patients with Rai stage III or stage IV disease, chromosome 11q22.3 deletion, and unmutated IGHV.
Study coauthor Jacqueline Claudia Barrientos, MD, associate professor at the Feinstein Institutes for Medical Research in New York, emphasized that the study does not provide any evidence that the addition of rituximab improved outcomes or remission duration beyond what is achieved by ibrutinib alone. Because the study didn’t include an ibrutinib-monotherapy arm, there’s no way to know. “I don’t believe that we should move to using combination ibrutinib plus rituximab for frontline except in specific cases, such as uncontrolled autoimmune complications or in a patient with a very aggressive disease that requires combined therapy because monotherapy is taking too long to work,” Dr Barrientos said.
She also pointed out that widespread use of ibrutinib as a frontline treatment would raise several issues. Although ibrutinib appears to cause less myelosuppression than FCR, she said, “there are still other issues that can affect quality of life.” These include bleeding, joint pain, diarrhea, cardiac arrhythmias, and other side effects. Dr Barrientos noted that because ibrutinib needs to be taken indefinitely, sorting out these complications is vital.
Cost is another concern. Again, the long-term nature of ibrutinib therapy is the issue: the drug may be too expensive to sustain treatment. In a 2015 paper in the Journal of Oncology Practice, Dr Shanafelt discussed the potential financial burden that would confront the movement toward novel targeted agents, such as ibrutinib, for frontline treatment of CLL.3 This shift, Dr Shanafelt wrote, “will dramatically increase individual out-of-pocket and societal costs of caring for patients with CLL. These cost considerations may undermine the potential promise of these agents by limiting access and reducing adherence.”
Nitin Jain, MD, associate professor of medicine at MD Anderson Cancer Center, Houston, Texas, who was not involved with the study, was optimistic about the findings. The results, he said, were not just statistically significant but also clinically significant. “This study establishes a new treatment paradigm for younger patients with CLL,” he says. However, he emphasized that this benefit is restricted to patients with IGVH-unmutated disease, per the study findings.
As Dr Jain sees it, the study is just one more piece of the puzzle showing how ibrutinib and new drugs may best benefit patients with CLL. “Several ongoing randomized studies are evaluating combinations of targeted therapies with chemoimmunotherapy,” Dr Jain noted. “The results of these studies will further help define the optimal frontline approach for patients with CLL.”
Disclosure: The study was supported by the National Cancer Institute and Pharmacyclics. Some of the authors of the study reported receiving payments from the pharmaceutical industry. For a full list of disclosures, please refer to the original study.
References
- Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437.
- Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib–Rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432-443.
- Shanafelt TD, Borah BJ, Finnes HD, et al. Impact of ibrutinib and idelalisib on the pharmaceutical cost of treating chronic lymphocytic leukemia at the individual and societal levels. J Oncol Pract. 2015;11(3):252-258.